Usage of Remdesivir for severe cases of corona approved in India!


Send us your feedback to audioarticles@vaarta.com



India has approved Gilead Sciences Inc's antiviral drug remdesivir for emergency usage in treating patients severely affected by the Coronavirus infection. It must be noted that Remdesivir is the first drug to show some improvement in Coronavirus patients in clinical trials.
Remdesivir has been granted emergency use authorisation by the US Food and Drug Administration in May and was also approved by Japanese health regulators. The Drugs Controller General of India has announced that Remdesivir was approved on June 1 under emergency use with condition being a five dose administration.
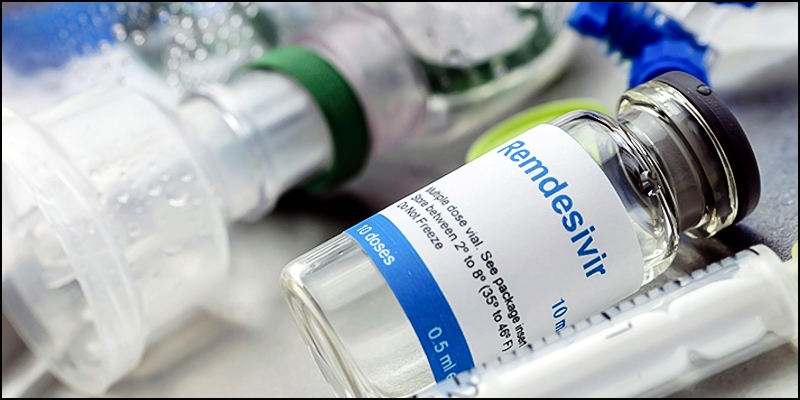
Gilead Sciences has reported that remdesivir displayed improvements in patients who had moderate Coronavirus infection, during a five-day dosage of the drug. European and South Korean countries are also planning to administer and authorize the usage of remdesivir for Coronavirus treatment.
Follow us on Google News and stay updated with the latest!
Comments
- logoutLogout

-

Devan Karthik
Contact at support@indiaglitz.com




 Follow
Follow






















-a3e.jpg)
-3c4.jpg)
-e5c.jpg)
-e66.jpg)
-71b.jpg)
-5d5.jpg)
-adc.jpg)
-798.jpg)

-7c2.jpg)





































